What Is The Shaped, Solid Mass That Is Used For Heavy Coverage And Is Also Known As Pancake Makeup?
Chapter 1. Essential Ideas
1.2 Phases and Classification of Matter
Learning Objectives
By the finish of this section, you will be able to:
- Draw the basic properties of each physical state of matter: solid, liquid, and gas
- Ascertain and requite examples of atoms and molecules
- Classify matter every bit an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical country and limerick
- Distinguish between mass and weight
- Apply the police force of conservation of matter
Thing is defined as anything that occupies space and has mass, and information technology is all around us. Solids and liquids are more obviously affair: We can see that they take up space, and their weight tells us that they have mass. Gases are also matter; if gases did not have upwardly space, a balloon would stay complanate rather than inflate when filled with gas.
Solids, liquids, and gases are the three states of matter commonly found on globe (Effigy ane). A solid is rigid and possesses a definite shape. A liquid flows and takes the shape of a container, except that it forms a flat or slightly curved upper surface when acted upon by gravity. (In zero gravity, liquids presume a spherical shape.) Both liquid and solid samples take volumes that are very near independent of pressure. A gas takes both the shape and volume of its container.
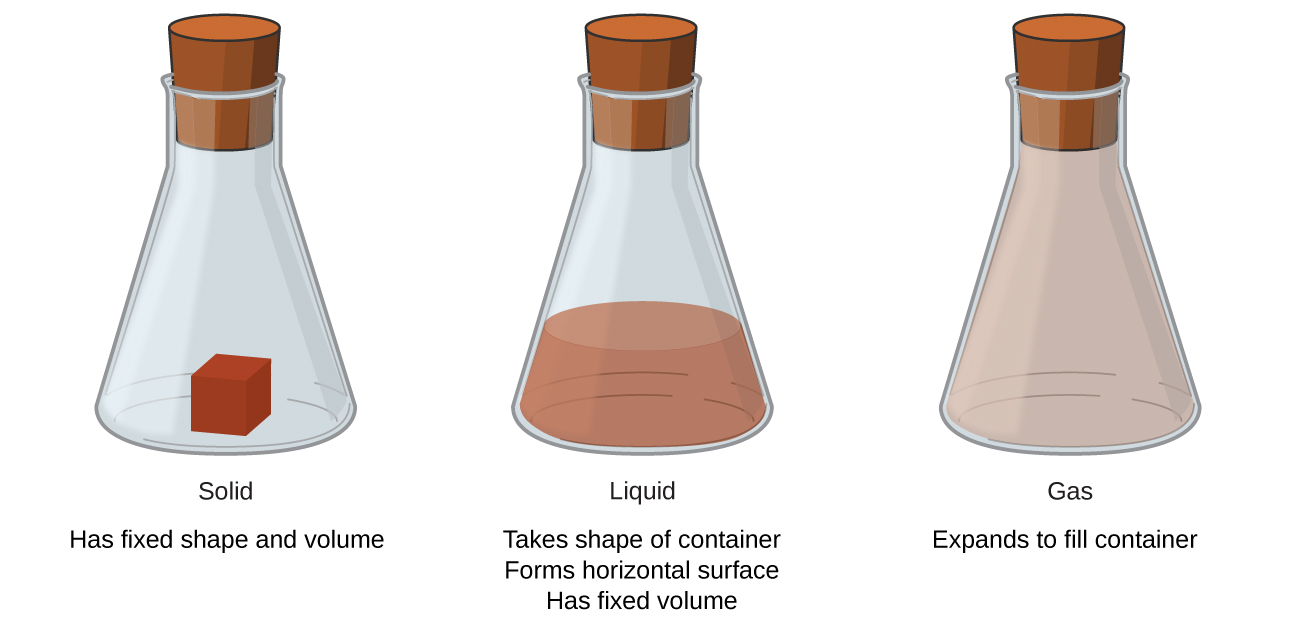
A fourth state of thing, plasma, occurs naturally in the interiors of stars. A plasma is a gaseous state of matter that contains observable numbers of electrically charged particles (Effigy 2). The presence of these charged particles imparts unique backdrop to plasmas that justify their classification equally a state of matter distinct from gases. In addition to stars, plasmas are found in some other high-temperature environments (both natural and man-made), such as lightning strikes, certain television screens, and specialized analytical instruments used to detect trace amounts of metals.


In a tiny jail cell in a plasma idiot box, the plasma emits ultraviolet light, which in turn causes the display at that location to announced a specific color. The composite of these tiny dots of color makes up the image that you lot see. Watch this video to acquire more about plasma and the places you encounter it.
Some samples of thing announced to have properties of solids, liquids, and/or gases at the same time. This can occur when the sample is composed of many small pieces. For example, we can pour sand as if it were a liquid because it is equanimous of many modest grains of solid sand. Thing can also have properties of more than one state when it is a mixture, such as with clouds. Clouds appear to behave somewhat like gases, merely they are actually mixtures of air (gas) and tiny particles of water (liquid or solid).
The mass of an object is a measure out of the corporeality of matter in it. One mode to measure out an object's mass is to measure the strength it takes to accelerate the object. It takes much more strength to accelerate a car than a bicycle because the motorcar has much more mass. A more mutual style to determine the mass of an object is to utilise a balance to compare its mass with a standard mass.
Although weight is related to mass, it is non the aforementioned affair. Weight refers to the force that gravity exerts on an object. This force is directly proportional to the mass of the object. The weight of an object changes equally the forcefulness of gravity changes, but its mass does not. An astronaut'southward mass does not modify just because she goes to the moon. But her weight on the moon is only i-sixth her globe-bound weight considering the moon'southward gravity is just ane-sixth that of the earth's. She may feel "weightless" during her trip when she experiences negligible external forces (gravitational or whatsoever other), although she is, of grade, never "massless."
The police of conservation of thing summarizes many scientific observations about matter: Information technology states that there is no detectable change in the total quantity of matter present when matter converts from one blazon to another (a chemical modify) or changes among solid, liquid, or gaseous states (a physical alter). Brewing beer and the operation of batteries provide examples of the conservation of thing (Figure 3). During the brewing of beer, the ingredients (water, yeast, grains, malt, hops, and sugar) are converted into beer (h2o, booze, carbonation, and flavoring substances) with no actual loss of substance. This is most clearly seen during the bottling process, when glucose turns into ethanol and carbon dioxide, and the full mass of the substances does non change. This can also be seen in a lead-acid car bombardment: The original substances (lead, lead oxide, and sulfuric acid), which are capable of producing electricity, are changed into other substances (atomic number 82 sulfate and water) that exercise non produce electricity, with no change in the bodily amount of thing.
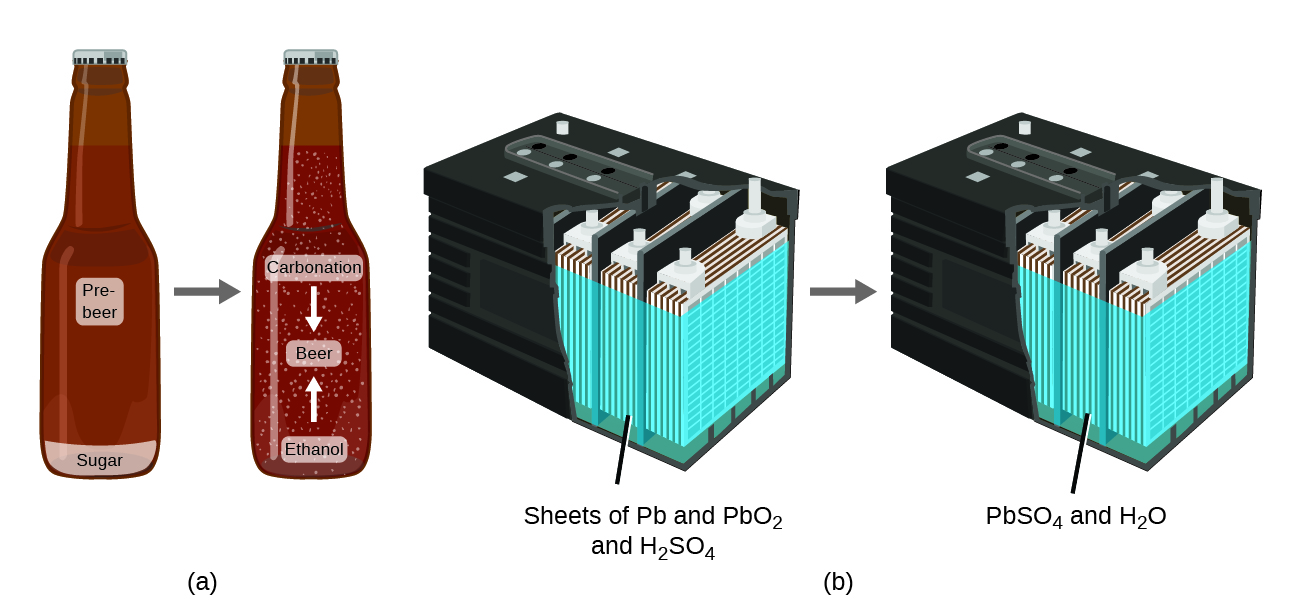
Although this conservation police force holds truthful for all conversions of matter, convincing examples are few and far between because, exterior of the controlled conditions in a laboratory, we seldom collect all of the cloth that is produced during a particular conversion. For instance, when y'all consume, digest, and assimilate food, all of the matter in the original food is preserved. But because some of the matter is incorporated into your body, and much is excreted every bit diverse types of waste material, it is challenging to verify by measurement.
Atoms and Molecules
An atom is the smallest particle of an chemical element that has the backdrop of that chemical element and tin can enter into a chemic combination. Consider the element gold, for example. Imagine cutting a gold nugget in half, then cutting one of the halves in half, and repeating this procedure until a piece of gold remained that was and so small that it could not exist cut in half (regardless of how tiny your knife may be). This minimally sized slice of gold is an atom (from the Greek atomos, meaning "indivisible") (Effigy 4). This atom would no longer be gold if it were divided whatsoever further.
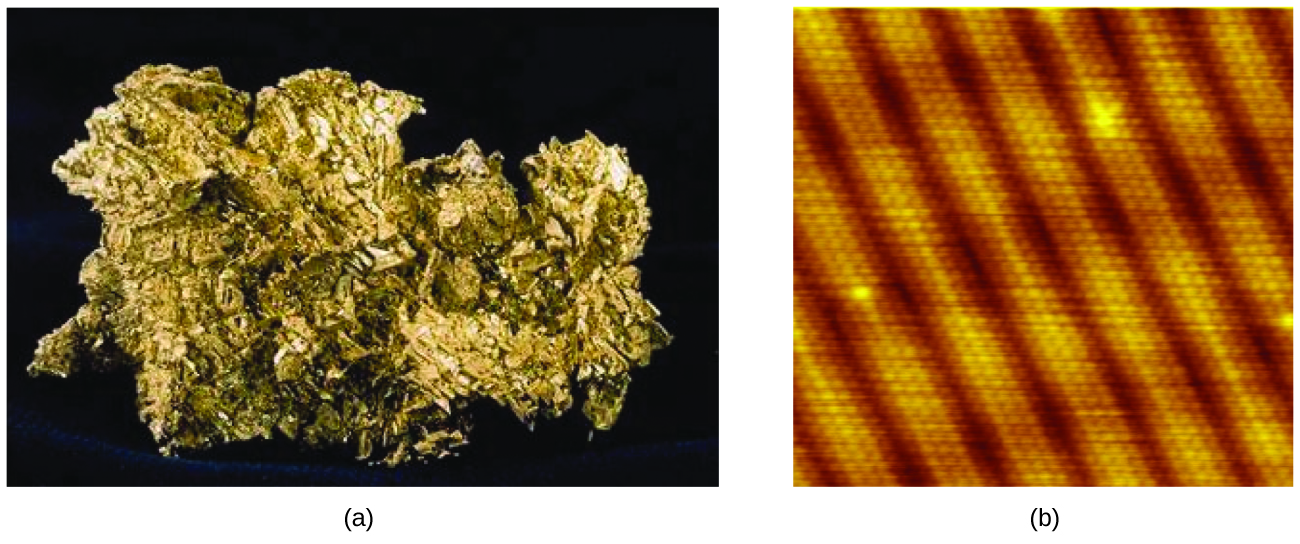
The kickoff proffer that matter is composed of atoms is attributed to the Greek philosophers Leucippus and Democritus, who developed their ideas in the fifth century BCE. Notwithstanding, information technology was not until the early on nineteenth century that John Dalton (1766–1844), a British schoolteacher with a great interest in science, supported this hypothesis with quantitative measurements. Since that time, repeated experiments accept confirmed many aspects of this hypothesis, and it has become one of the central theories of chemistry. Other aspects of Dalton'southward diminutive theory are still used merely with minor revisions (details of Dalton's theory are provided in the affiliate on atoms and molecules).
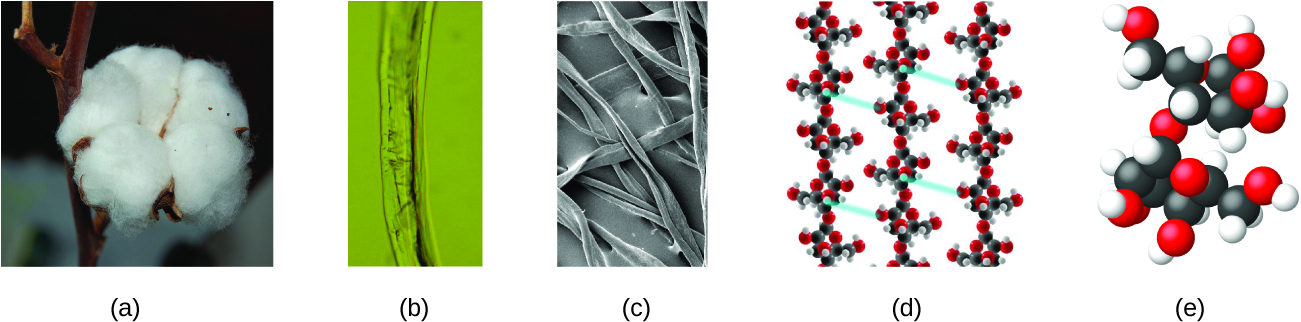
An atom is so minor that its size is difficult to imagine. I of the smallest things nosotros can see with our unaided eye is a unmarried thread of a spider web: These strands are nigh ane/10,000 of a centimeter (0.0001 cm) in diameter. Although the cross-department of i strand is almost impossible to come across without a microscope, it is huge on an atomic calibration. A unmarried carbon atom in the web has a diameter of well-nigh 0.000000015 centimeter, and it would take near 7000 carbon atoms to bridge the diameter of the strand. To put this in perspective, if a carbon atom were the size of a dime, the cantankerous-section of one strand would be larger than a football game field, which would require virtually 150 million carbon cantlet "dimes" to encompass it. (Effigy 5) shows increasingly close microscopic and atomic-level views of ordinary cotton.
An atom is and then low-cal that its mass is also difficult to imagine. A billion lead atoms (1,000,000,000 atoms) counterbalance about three × 10−xiii grams, a mass that is far too light to exist weighed on even the earth's most sensitive balances. It would crave over 300,000,000,000,000 atomic number 82 atoms (300 trillion, or iii × ten14) to be weighed, and they would weigh only 0.0000001 gram.
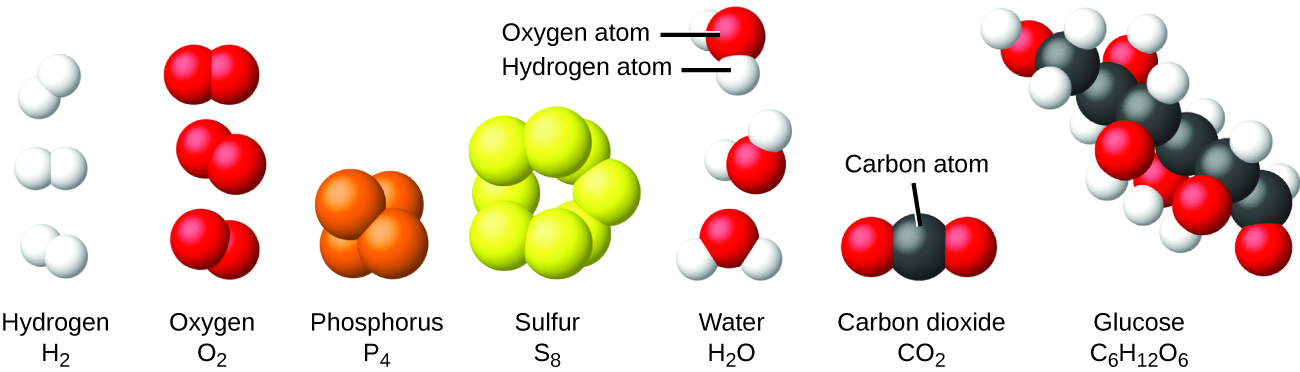
It is rare to find collections of individual atoms. Only a few elements, such every bit the gases helium, neon, and argon, consist of a drove of individual atoms that move about independently of ane another. Other elements, such as the gases hydrogen, nitrogen, oxygen, and chlorine, are composed of units that consist of pairs of atoms (Effigy half dozen). One form of the element phosphorus consists of units composed of iv phosphorus atoms. The element sulfur exists in various forms, one of which consists of units equanimous of eight sulfur atoms. These units are called molecules. A molecule consists of 2 or more atoms joined by potent forces called chemical bonds. The atoms in a molecule movement around every bit a unit of measurement, much like the cans of soda in a six-pack or a bunch of keys joined together on a unmarried cardinal band. A molecule may consist of two or more identical atoms, every bit in the molecules found in the elements hydrogen, oxygen, and sulfur, or information technology may consist of ii or more than different atoms, as in the molecules found in h2o. Each water molecule is a unit that contains two hydrogen atoms and one oxygen atom. Each glucose molecule is a unit of measurement that contains vi carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. Similar atoms, molecules are incredibly small and lite. If an ordinary glass of h2o were enlarged to the size of the earth, the water molecules inside information technology would be nearly the size of golf balls.
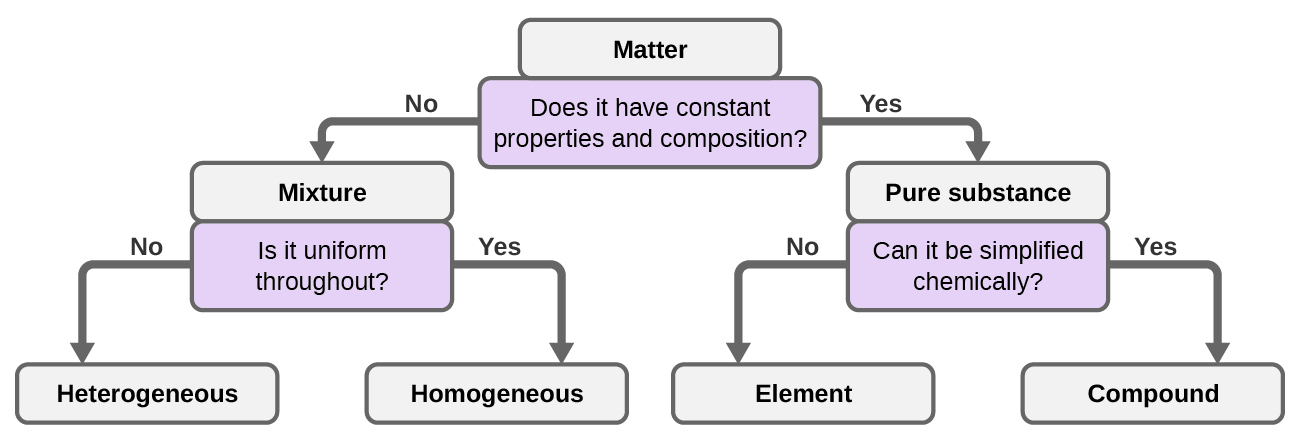
Classifying Matter
We can classify thing into several categories. Two wide categories are mixtures and pure substances. A pure substance has a abiding limerick. All specimens of a pure substance have exactly the aforementioned makeup and backdrop. Any sample of sucrose (table saccharide) consists of 42.1% carbon, vi.5% hydrogen, and 51.4% oxygen by mass. Whatsoever sample of sucrose besides has the same physical properties, such every bit melting point, color, and sweetness, regardless of the source from which it is isolated.
We can divide pure substances into two classes: elements and compounds. Pure substances that cannot be broken down into simpler substances past chemical changes are called elements. Iron, silver, aureate, aluminum, sulfur, oxygen, and copper are familiar examples of the more 100 known elements, of which about 90 occur naturally on the world, and two dozen or and so have been created in laboratories.
Pure substances that tin be broken down by chemical changes are chosen compounds. This breakdown may produce either elements or other compounds, or both. Mercury(2) oxide, an orange, crystalline solid, can be broken down by oestrus into the elements mercury and oxygen (Figure 7). When heated in the absence of air, the compound sucrose is cleaved down into the element carbon and the compound h2o. (The initial phase of this process, when the sugar is turning brown, is known as caramelization—this is what imparts the characteristic sweet and nutty season to caramel apples, caramelized onions, and caramel). Silverish(I) chloride is a white solid that can be cleaved downwardly into its elements, argent and chlorine, past absorption of calorie-free. This property is the ground for the use of this compound in photographic films and photochromic eyeglasses (those with lenses that darken when exposed to light).
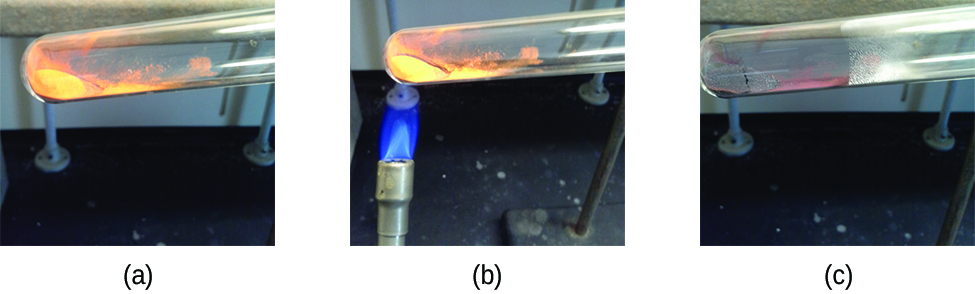

Many compounds interruption down when heated. This site shows the breakdown of mercury oxide, HgO. You can also view an example of the photochemical decomposition of silver chloride (AgCl), the basis of early photography.
The properties of combined elements are different from those in the free, or uncombined, state. For example, white crystalline saccharide (sucrose) is a compound resulting from the chemical combination of the element carbon, which is a blackness solid in one of its uncombined forms, and the two elements hydrogen and oxygen, which are colorless gases when uncombined. Costless sodium, an element that is a soft, shiny, metallic solid, and free chlorine, an element that is a yellow-light-green gas, combine to form sodium chloride (table salt), a compound that is a white, crystalline solid.
A mixture is equanimous of two or more than types of matter that tin can exist present in varying amounts and can be separated past physical changes, such every bit evaporation (you will learn more about this later). A mixture with a composition that varies from point to point is called a heterogeneous mixture. Italian dressing is an instance of a heterogeneous mixture (Figure 8). Its composition can vary considering we can make information technology from varying amounts of oil, vinegar, and herbs. It is not the same from point to point throughout the mixture—one drop may be mostly vinegar, whereas a different drop may be mostly oil or herbs because the oil and vinegar carve up and the herbs settle. Other examples of heterogeneous mixtures are chocolate chip cookies (we tin see the divide bits of chocolate, basics, and cookie dough) and granite (we can see the quartz, mica, feldspar, and more).
A homogeneous mixture, likewise called a solution, exhibits a uniform limerick and appears visually the same throughout. An case of a solution is a sports drink, consisting of h2o, sugar, coloring, flavoring, and electrolytes mixed together uniformly (Figure 8). Each drop of a sports drink tastes the same because each drib contains the aforementioned amounts of water, sugar, and other components. Annotation that the limerick of a sports drink can vary—it could be made with somewhat more or less saccharide, flavoring, or other components, and notwithstanding be a sports drink. Other examples of homogeneous mixtures include air, maple syrup, gasoline, and a solution of salt in water.

Although in that location are just over 100 elements, tens of millions of chemical compounds result from different combinations of these elements. Each compound has a specific composition and possesses definite chemic and physical properties by which we can distinguish it from all other compounds. And, of grade, at that place are innumerable ways to combine elements and compounds to form dissimilar mixtures. A summary of how to distinguish between the various major classifications of thing is shown in (Figure ix).
Eleven elements make up about 99% of the earth's crust and atmosphere (Table 1). Oxygen constitutes nearly one-half and silicon most one-quarter of the total quantity of these elements. A majority of elements on earth are constitute in chemic combinations with other elements; about one-quarter of the elements are as well establish in the free state.
| Chemical element | Symbol | Percentage Mass | Chemical element | Symbol | Percentage Mass | |
|---|---|---|---|---|---|---|
| oxygen | O | 49.xx | chlorine | Cl | 0.19 | |
| silicon | Si | 25.67 | phosphorus | P | 0.11 | |
| aluminum | Al | 7.50 | manganese | Mn | 0.09 | |
| iron | Fe | iv.71 | carbon | C | 0.08 | |
| calcium | Ca | three.39 | sulfur | S | 0.06 | |
| sodium | Na | 2.63 | barium | Ba | 0.04 | |
| potassium | K | 2.twoscore | nitrogen | North | 0.03 | |
| magnesium | Mg | ane.93 | fluorine | F | 0.03 | |
| hydrogen | H | 0.87 | strontium | Sr | 0.02 | |
| titanium | Ti | 0.58 | all others | – | 0.47 | |
| Table ane. Elemental Composition of Earth | ||||||
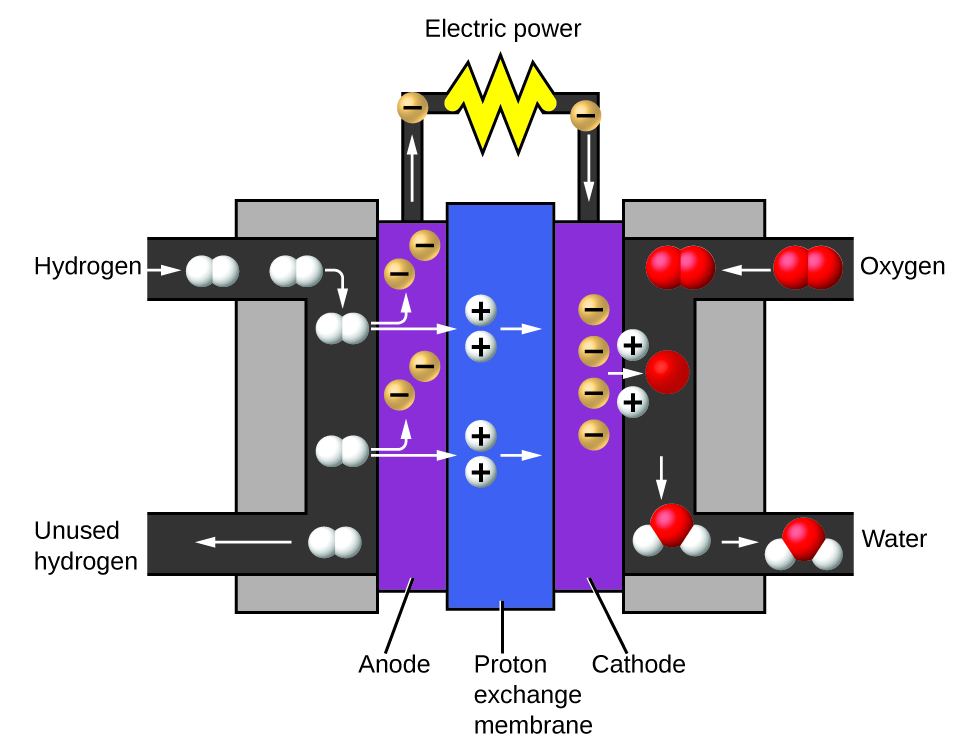
Decomposition of H2o / Production of Hydrogen
Water consists of the elements hydrogen and oxygen combined in a 2 to one ratio. H2o tin can exist broken downwards into hydrogen and oxygen gases by the addition of energy. One way to do this is with a battery or power supply, equally shown in (Figure 10).
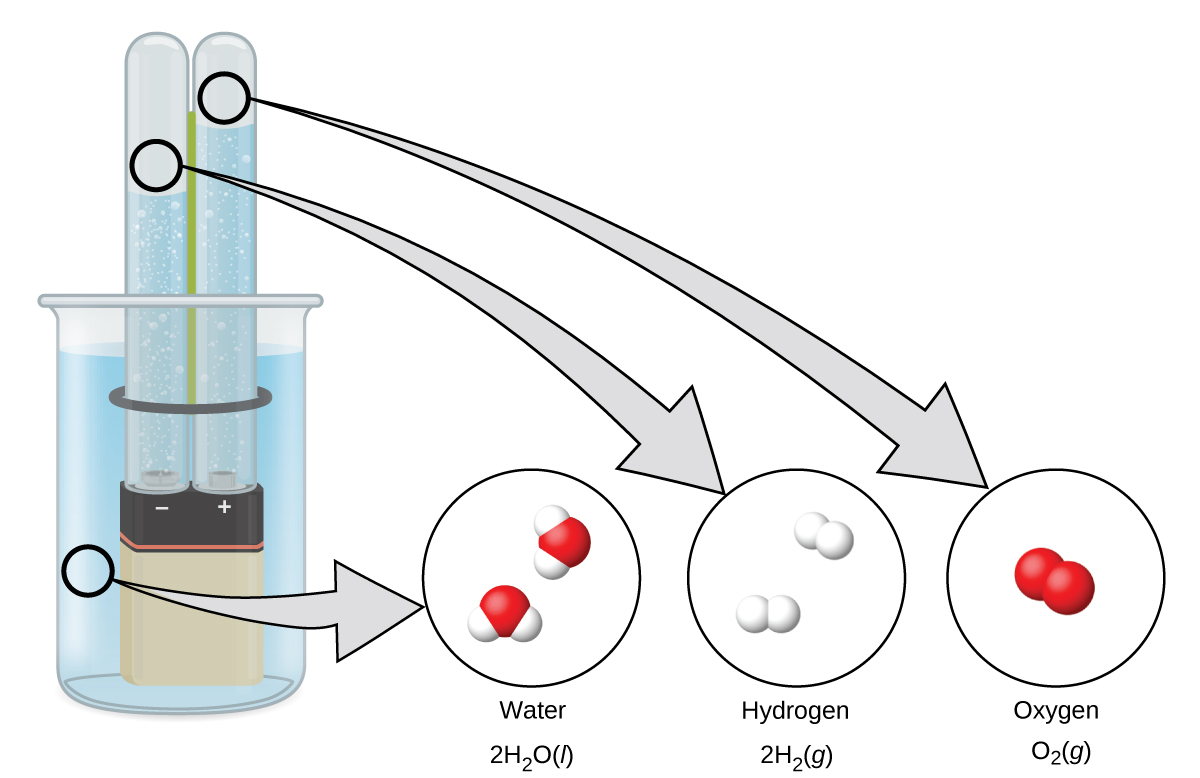
The breakdown of water involves a rearrangement of the atoms in water molecules into unlike molecules, each composed of two hydrogen atoms and two oxygen atoms, respectively. Two water molecules form one oxygen molecule and two hydrogen molecules. The representation for what occurs, [latex]ii\text{H}_2\text{O}(l) \rightarrow ii\text{H}_2(g) + \text{O}_2(g)[/latex], will be explored in more than depth in later chapters.
The two gases produced take distinctly unlike properties. Oxygen is non combustible but is required for combustion of a fuel, and hydrogen is highly flammable and a potent free energy source. How might this knowledge be applied in our world? I awarding involves research into more than fuel-efficient transportation. Fuel-cell vehicles (FCV) run on hydrogen instead of gasoline (Figure 11). They are more efficient than vehicles with internal combustion engines, are nonpolluting, and reduce greenhouse gas emissions, making us less dependent on fossil fuels. FCVs are not yet economically viable, however, and current hydrogen production depends on natural gas. If we can develop a process to economically decompose water, or produce hydrogen in another environmentally audio way, FCVs may exist the way of the futurity.
Chemistry of Cell Phones
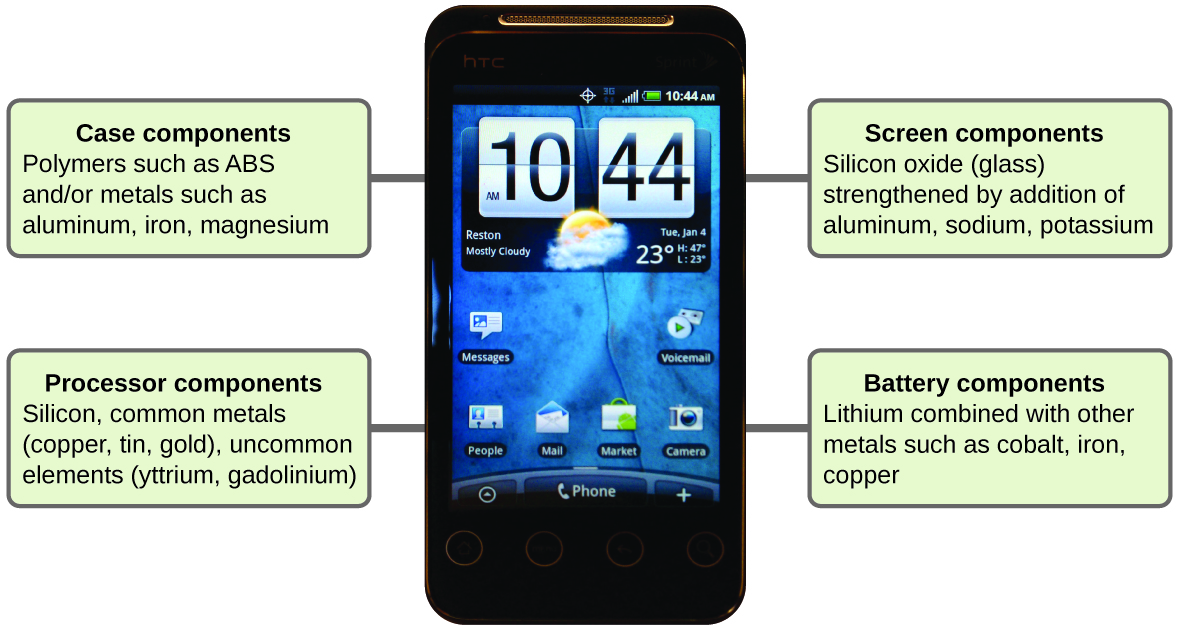
Imagine how different your life would be without cell phones (Figure 12) and other smart devices. Cell phones are fabricated from numerous chemical substances, which are extracted, refined, purified, and assembled using an extensive and in-depth understanding of chemical principles. About xxx% of the elements that are found in nature are found within a typical smart phone. The case/body/frame consists of a combination of sturdy, durable polymers comprised primarily of carbon, hydrogen, oxygen, and nitrogen [acrylonitrile butadiene styrene (ABS) and polycarbonate thermoplastics], and light, strong, structural metals, such as aluminum, magnesium, and iron. The brandish screen is made from a specially toughened glass (silica glass strengthened by the addition of aluminum, sodium, and potassium) and coated with a material to make it conductive (such as indium tin oxide). The circuit board uses a semiconductor material (usually silicon); unremarkably used metals similar copper, tin, silver, and golden; and more unfamiliar elements such as yttrium, praseodymium, and gadolinium. The battery relies upon lithium ions and a diversity of other materials, including iron, cobalt, copper, polyethylene oxide, and polyacrylonitrile.
Fundamental Concepts and Summary
Matter is anything that occupies infinite and has mass. The bones building block of matter is the cantlet, the smallest unit of an element that tin enter into combinations with atoms of the same or other elements. In many substances, atoms are combined into molecules. On globe, matter commonly exists in iii states: solids, of fixed shape and volume; liquids, of variable shape but fixed book; and gases, of variable shape and volume. Under high-temperature conditions, thing besides can exist as a plasma. Nigh matter is a mixture: Information technology is composed of two or more types of matter that can be present in varying amounts and tin exist separated by concrete ways. Heterogeneous mixtures vary in composition from point to point; homogeneous mixtures take the aforementioned limerick from point to point. Pure substances consist of merely one type of thing. A pure substance tin can exist an element, which consists of only one type of atom and cannot be broken downward by a chemical change, or a compound, which consists of two or more types of atoms.
Chemistry Terminate of Chapter Exercises
- Why practise we use an object's mass, rather than its weight, to indicate the amount of matter it contains?
- What backdrop distinguish solids from liquids? Liquids from gases? Solids from gases?
- How does a heterogeneous mixture differ from a homogeneous mixture? How are they similar?
- How does a homogeneous mixture differ from a pure substance? How are they similar?
- How does an chemical element differ from a compound? How are they similar?
- How practise molecules of elements and molecules of compounds differ? In what ways are they similar?
- How does an atom differ from a molecule? In what ways are they like?
- Many of the items you purchase are mixtures of pure compounds. Select three of these commercial products and prepare a list of the ingredients that are pure compounds.
- Classify each of the following every bit an chemical element, a compound, or a mixture:
(a) copper
(b) water
(c) nitrogen
(d) sulfur
(eastward) air
(f) sucrose
(g) a substance composed of molecules each of which contains two iodine atoms
(h) gasoline
- Classify each of the following as an element, a compound, or a mixture:
(a) atomic number 26
(b) oxygen
(c) mercury oxide
(d) pancake syrup
(east) carbon dioxide
(f) a substance composed of molecules each of which contains one hydrogen atom and ane chlorine cantlet
(grand) blistering soda
(h) baking powder
- A sulfur atom and a sulfur molecule are not identical. What is the difference?
- How are the molecules in oxygen gas, the molecules in hydrogen gas, and h2o molecules similar? How do they differ?
- We refer to astronauts in space as weightless, but not without mass. Why?
- As we drive an automobile, nosotros don't think about the chemicals consumed and produced. Prepare a list of the main chemicals consumed and produced during the operation of an automobile.
- Matter is everywhere around us. Brand a list by name of fifteen different kinds of affair that you encounter every day. Your list should include (and label at least 1 instance of each) the following: a solid, a liquid, a gas, an element, a chemical compound, a homogenous mixture, a heterogeneous mixture, and a pure substance.
- When elemental iron corrodes it combines with oxygen in the air to ultimately form cerise brownish atomic number 26(III) oxide which we call rust. (a) If a shiny iron blast with an initial mass of 23.2 g is weighed after being coated in a layer of rust, would you wait the mass to have increased, decreased, or remained the same? Explain. (b) If the mass of the iron nail increases to 24.1 g, what mass of oxygen combined with the iron?
- As stated in the text, disarming examples that demonstrate the law of conservation of thing exterior of the laboratory are few and far between. Bespeak whether the mass would increase, subtract, or stay the same for the following scenarios where chemical reactions accept place:
(a) Exactly 1 pound of bread dough is placed in a baking tin. The dough is cooked in an oven at 350 °F releasing a wonderful aroma of freshly baked bread during the cooking process. Is the mass of the baked loaf less than, greater than, or the same every bit the one pound of original dough? Explain.
(b) When magnesium burns in air a white flaky ash of magnesium oxide is produced. Is the mass of magnesium oxide less than, greater than, or the same as the original slice of magnesium? Explain.
(c) Antoine Lavoisier, the French scientist credited with start stating the law of conservation of matter, heated a mixture of tin and air in a sealed flask to produce can oxide. Did the mass of the sealed flask and contents subtract, increase, or remain the aforementioned after the heating?
- Yeast converts glucose to ethanol and carbon dioxide during anaerobic fermentation as depicted in the simple chemical equation here:
[latex]\text{glucose} \rightarrow \text{ethanol + carbon dioxide}[/latex]
(a) If 200.0 chiliad of glucose is fully converted, what will exist the total mass of ethanol and carbon dioxide produced?
(b) If the fermentation is carried out in an open container, would you expect the mass of the container and contents after fermentation to exist less than, greater than, or the aforementioned as the mass of the container and contents earlier fermentation? Explicate.
(c) If 97.vii g of carbon dioxide is produced, what mass of ethanol is produced?
Glossary
- atom
- smallest particle of an element that tin enter into a chemical combination
- chemical compound
- pure substance that can be decomposed into two or more elements
- chemical element
- substance that is composed of a single blazon of atom; a substance that cannot exist decomposed by a chemical change
- gas
- country in which matter has neither definite volume nor shape
- heterogeneous mixture
- combination of substances with a composition that varies from point to point
- homogeneous mixture
- (also, solution) combination of substances with a limerick that is compatible throughout
- liquid
- state of matter that has a definite volume but indefinite shape
- law of conservation of matter
- when affair converts from 1 type to another or changes grade, there is no detectable change in the total amount of matter present
- mass
- fundamental property indicating amount of matter
- affair
- annihilation that occupies space and has mass
- mixture
- thing that can be separated into its components past physical ways
- molecule
- bonded collection of ii or more atoms of the same or different elements
- plasma
- gaseous state of matter containing a large number of electrically charged atoms and/or molecules
- pure substance
- homogeneous substance that has a constant limerick
- solid
- state of thing that is rigid, has a definite shape, and has a fairly constant book
- weight
- force that gravity exerts on an object
Solutions
Answers for Chemical science End of Chapter Exercises
2. Liquids can change their shape (flow); solids can't. Gases tin can undergo large volume changes as force per unit area changes; liquids do not. Gases flow and change volume; solids do not.
4. The mixture can have a variety of compositions; a pure substance has a definite composition. Both have the same composition from point to point.
6. Molecules of elements contain just one blazon of atom; molecules of compounds contain two or more than types of atoms. They are similar in that both are comprised of two or more than atoms chemically bonded together.
8. Answers will vary. Sample answer: Gatorade contains water, sugar, dextrose, citric acid, salt, sodium chloride, monopotassium phosphate, and sucrose acetate isobutyrate.
10. (a) chemical element; (b) chemical element; (c) compound; (d) mixture, (e) compound; (f) compound; (g) compound; (h) mixture
12. In each case, a molecule consists of two or more combined atoms. They differ in that the types of atoms alter from one substance to the side by side.
14. Gasoline (a mixture of compounds), oxygen, and to a lesser extent, nitrogen are consumed. Carbon dioxide and water are the main products. Carbon monoxide and nitrogen oxides are produced in bottom amounts.
16. (a) Increased as it would accept combined with oxygen in the air thus increasing the corporeality of matter and therefore the mass. (b) 0.9 1000
18. (a) 200.0 one thousand; (b) The mass of the container and contents would decrease as carbon dioxide is a gaseous product and would leave the container. (c) 102.3 grand
Source: https://opentextbc.ca/chemistry/chapter/phases-and-classification-of-matter/
Posted by: stewartfortalwyneho.blogspot.com

0 Response to "What Is The Shaped, Solid Mass That Is Used For Heavy Coverage And Is Also Known As Pancake Makeup?"
Post a Comment